Harman's Compounds
 This
page presents the compound polyhedra which are described in Michael G.
Harman's interesting but unpublished 1974 paper "Polyhedral Compounds,"
cited in the references. Most of these polyhedra
had never been described before or since, and many have never been made
as paper models or otherwise illustrated. As background, one should be
familiar with the page about compounds.
Also, some of these fall under the category compounds
of cubes and/or uniform compounds.
This
page presents the compound polyhedra which are described in Michael G.
Harman's interesting but unpublished 1974 paper "Polyhedral Compounds,"
cited in the references. Most of these polyhedra
had never been described before or since, and many have never been made
as paper models or otherwise illustrated. As background, one should be
familiar with the page about compounds.
Also, some of these fall under the category compounds
of cubes and/or uniform compounds.
Harman's general procedure can be paraphrased as follows:
- Choose a polyhedron to make a compound from and an overall symmetry group to attain. Think of their symmetries and pick some n and m for which the component has an n-fold axis of symmetry and the overall compound has an m-fold axis.
- Imagine the symmetry axes of the compound as radiating from a point in space and visualize one polyhedral component centered on that point, turned so one of its n-fold symmetry axes aligns with an m-fold symmetry axis of the compound.
- Imagine rotating the component on the axis so that each of the m planes of symmetry of the compound that pass through the axis has some plane of symmetry of the component coincident with it. If n is not divisible by m, this can not be achieved with a single copy of the component along the axis, in which case m copies along the same axis are first superimposed. Sometimes there is more than one angle which covers the m symmetry planes, and then different variants of a compound exist.
- For every m-fold axis of the desired compound, superimpose a copy (or m copies) of the component with an n-fold axis so oriented.
- Check to see if the components so placed will overlap so that the number of components needed is less than the number of m-fold axes.
Harman uses the notation nX/mY to indicate that an n-fold axis of the X symmetry group in the component is placed along the direction of the m-fold axis of the Y symmetry group of the compound. X and Y are i, c, or t, for the icosahedral, cuboctahedral, or tetrahedral symmetries, respectively.
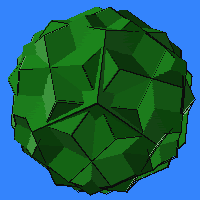
 Icosahedral
Symmetry of Compound:
Icosahedral
Symmetry of Compound:
- 6 icosahedra, 6 dodecahedra, 5i/5i.
- 10 icosahedra, 10 dodecahedra, 3i/3i.
- 5 icosahedra, 5 dodecahedra, 2i/2i, (although there are fifteen 2-fold axes, groups of three components coincide, leaving only five distinct components.)
- 10 cubes, 10 octahedra, 3c/3i, version A.
- 10 cubes, 10 octahedra, 3c/3i, version B.
- 5 cubes, 5 octahedra , 4c/2i, (these are the standard uniform compounds; again, fifteen overlap to become five.)
- 15 cubes, 15 octahedra, 2c/2i. (This could be derived from 4c/2i as a second variant.)
- 20 tetrahedra, 3t/3i, version A.
- 20 tetrahedra, 3t/3i, version B.
- 30 tetrahedra, 2t/2i.
Icosahedral symmetry of component:
This first group of examples was detailed on the bottom of the page about compounds:Octahedral symmetry of component:
This group of examples was detailed on the page about compounds of cubes:Tetrahedral symmetry of component:
The following are equivalent to substituting stellae octangula into the above compounds of cubes. Note that the familiar compounds of five and ten tetrahedra do not show up by this technique of aligning planes of symmetry.Octahedral Symmetry of Compound:
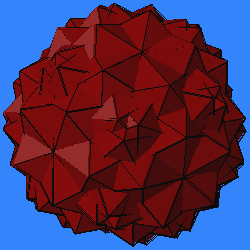
- 2 icosahedra, 2 dodecahedra, 2i/4c.
- 4 icosahedra, 4 dodecahedra, 3i/3c, version A.
- 4 icosahedra, 4 dodecahedra, 3i/3c, version B.
- 6 icosahedra, 6 dodecahedra, 2i/2c. (This can also be derived from 2i/4c as a second variant.)
- 3 cubes, 3 octahedra, 4c/4c.
- 4 cubes, 4 octahedra, 3c/3c.
- 6 cubes, 6 octahedra, 2c/2c.
- 2 tetrahedra, 3t/3c.
- 8 tetrahedra, 3t/3c.
- 6 tetrahedra, 2t/4c.
- 12 tetrahedra, 2t/2c.
Icosahedral symmetry of component:
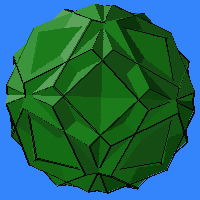 Note
how the following four correspond to the above icosahedral compounds of
octahedral components. The number of n-fold axes depends on the
overall symmetry, but the number of varieties agrees. This relation when
one "interchanges the role of component symmetry and overall symmetry"
is called outer duality by Harman. Shown at right is the 4 dodecahedra
version A, and at the top of the page is version B.
Note
how the following four correspond to the above icosahedral compounds of
octahedral components. The number of n-fold axes depends on the
overall symmetry, but the number of varieties agrees. This relation when
one "interchanges the role of component symmetry and overall symmetry"
is called outer duality by Harman. Shown at right is the 4 dodecahedra
version A, and at the top of the page is version B.
Octahedral symmetry of component:
These are the three rigid octahedral compounds listed on the page about compounds of cubes:Tetrahedral symmetry of component:
The following can also be seen as following from the above compounds of cubes (or, in the first case, a single cube) by substituting stellae octangula.
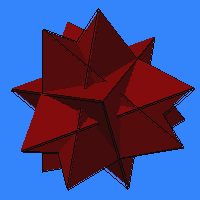 Tetrahedral
Symmetry of Compound:
Tetrahedral
Symmetry of Compound:
- 4 tetrahedra, 3t/3t.
Tetrahedral symmetry of component:
There is only one compound with tetrahedral symmetry on Harman's list. A beautiful compound of four tetrahedra, I like to call it the "stella sixteengula". If you combine it with its dual, you get the compound of 8 tetrahedra listed above.Prism Symmetry of Compound:
in progress...
Harman's examples are all rigid compounds of Platonic solids, but the idea is straightforward to apply to other symmetric polyhedra as components, e.g., a compound of five rhombic triacontahedra. Some examples with stellated components were listed elsewhere. The idea of nonrigid solids with rotational freedom which appears in Skilling's 1976 enumeration of uniform compounds is excluded by Harman's definitions. Most of the well known compounds can be made by this technique but the compounds of five tetrahedra and ten tetrahedra are not, because they have no planes of symmetry of the compound aligned with planes of symmetry of the components.
Note: Of course, under usual circumstances I would never consider presenting the results of someone else's unpublished paper without their expressed invitation. However, since Michael Harman's 1974 paper has remained unpublished for over twenty years, and since these compounds are so beautiful that they really deserve to be known, I feel it is appropriate for me to present them here in this manner. The reader, of course, understands that the credit for the general method and the first envisionment of these compounds belongs to Harman, and his unpublished paper should be cited as their original source.